We thank the following funders, past and present, for funding our research work:

We are a catalysis and synthesis group with particular interest in the following areas:
1. Gold Catalysis
In the past decade, gold has emerged as a powerful catalyst for the electrophilic activation of carbon-carbon π bonds. Gold catalysis represents a new frontier in catalysis with scope for further discoveries and development, particularly in the field of asymmetric gold catalysis. We are interested in the development of novel gold catalysed reactions with the aim of expanding the current toolkit of synthetic techniques. Recent highlights from our group include developing novel gold-catalysed reactions with cyclopropenes, allenes and allylic alcohols.
For representative publications, see: Org. Lett., 2020, 22, 6977 Abstract., ACS Catal., 2019, 9, 2552. Abstract; Chem. Eur. J., 2018, 24, 7002. Abstract; Chem. Eur. J., 2018, 24, 937. Article; Chem. Eur. J. 2016, 22, 18593. Article; Chem. Eur. J., 2015, 21, 13748. Article; J. Org. Chem., 2015, 80, 9807. Article; Chem. Commun., 2013, 49, 4262. Abstract; Org. Lett., 2012, 14, 898. Abstract; Chem. Commun.,2011, 47, 1333. Abstract; Org. Lett. 2010, 12, 484. Abstract; Chem. Commun., 2008, 6405. Abstract
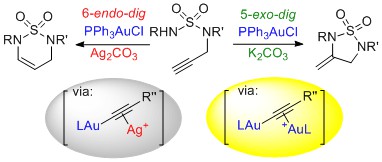
2. Dual Metal- and Photoredox-Catalysis
We have recently developed dual gold- and photoredox-catalysed methods for cross couplings through C-H activation or transmetallations. For representative publications, see: Chem. Sci. 2017, 8, 2885. Article; Chem. Commun. 2016, 52, 10163. Article. For our work on dual copper and photoredox-catalysed reactions, see: Chem. Commun, 2019, 55, 4238. Abstract and review: Tetrahedron, 2018, 74, 4881. Abstract.
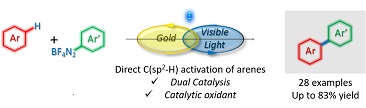
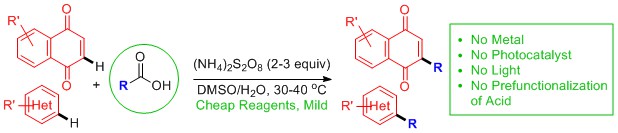
3. Decarboxylative Radical Reactions
We have recently developed Metal-, Photocatalyst-, and Light-Free Direct CH Functionalisations of Heterocycles. For representative publications: Org. Lett., 2019, 21, 7119. Abstract. Org. Lett., 2018, 20, 6863. Abstract. Org. Lett. 2022, 24, 686. Article. Org. Lett. 2022, 24, 43, 8008. Article.
4. Palladium Catalysis
We have recently developed a mild and ligand free cationic Pd(II) system for conjugate additions to hindered γ-substituted cyclohexenones. We have also developed a ligand- and base-free Pd(II)-Catalysed Controlled Switching between oxidative Heck and conjugate addition reactions, and controlled C-H functionalisations of benzoquinones. Current work in the group centres around the development and further applications of the cationic Pd(II) system as well as enantioselective Pd(II) reactions.
For representative publications: Org. Lett., 2019, 21, 8689. Abstract. Chem. Eur. J., 2017, 23, 18282. Article; Chem. Commun, 2015, 51, 4089. Article; Angew. Chem. Int. Ed., 2014, 53, 13876. Article; Org. Lett.,2013, 15, 1886. Abstract; Org. Lett., 2012, 14, 2508. Abstract. Review on Oxidative Boron Heck: Org. Biomol. Chem., 2016, 14, 5357. Article.
5. New gold catalysts:
Mesoionic N-Heterocyclic Carbene (NHC) Complexes
In collaboration with Dr James Crowley (University of Otago), we are studying novel gold(I) ‘‘click’’ carbene(1,2,3-triazolylidene) complexes and their applicationsin catalysis. For representative publication, see: Chem. Commun., 2011, 47, 328. Abstract; Organometallics., 2013, 32, 7065-7076. Article and review: Aust. J. Chem. 2011, 64, 1118. Abstract
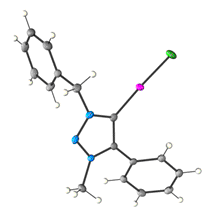
Gold(III)-oxo complexes
For representative publication, see: Catal. Sci. Technol., 2012, 2, 1818. Abstract
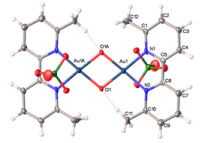
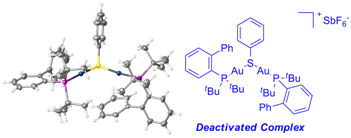
Deactivation of catalysts
For representative publication, see: see: Dalton Trans., 2013, 42, 9645-9653. Abstract